
Analysis of Ammonia -Water (NH3-H2O) Vapor Absorption Refrigeration System based on First Law of Thermodynamics | Semantic Scholar
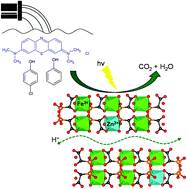
NH3/H2O-mediated proton conductivity and photocatalytic behaviour of Fe(ii)-hydroxyphosphonoacetate and M(ii)-substituted derivatives - Dalton Transactions (RSC Publishing)

Ammonia Solution, buy Price of industiral liquor ammonia NH4OH /NH3H2O on China Suppliers Mobile - 131790475

NH3⋅H2O: The Simplest Nitrogen‐Containing Ligand for Selective Aerobic Alcohol Oxidation to Aldehydes or Nitriles in Neat Water - Zhang - 2018 - ChemistryOpen - Wiley Online Library

IJMS | Free Full-Text | Effect of Ammonia on the Gas-Phase Hydration of the Common Atmospheric Ion HSO4-
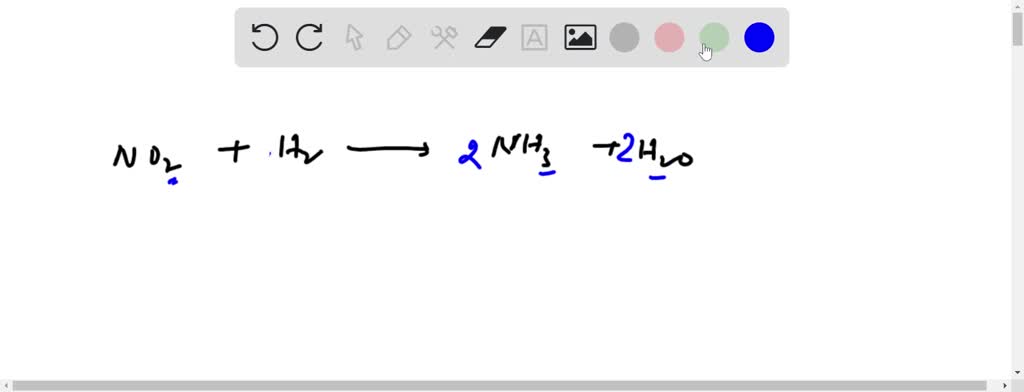
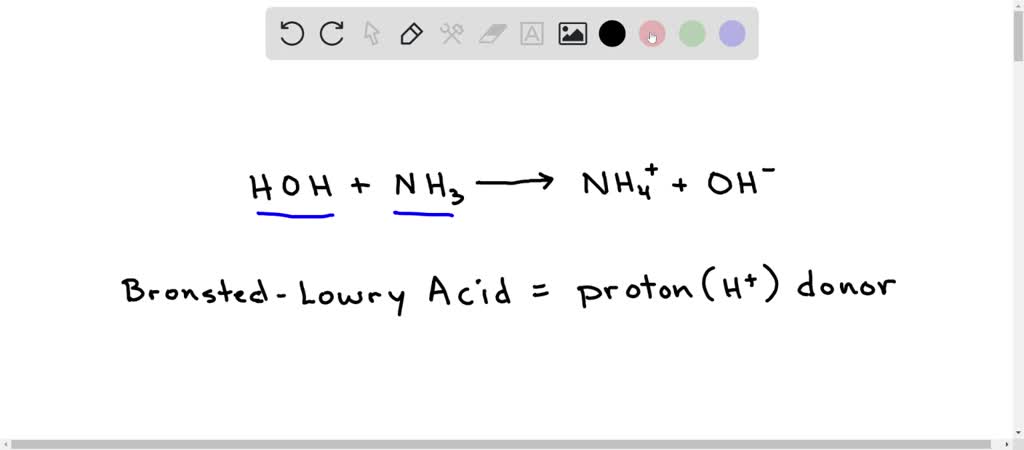
SOLVED: Balance the following oxidation-reduction reactions in acidic solution: NO2 + H2 —> NH3 + H2O

Arrange NH3,H2O and HF in the order of increasing magnitude of hydrogen bonding and explain the basis for your arrangement.
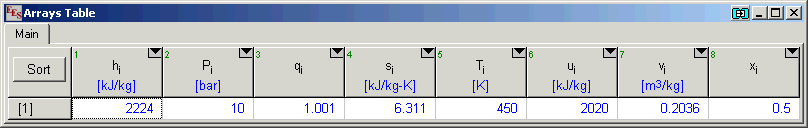
![PDF] A Helmholtz free energy equation of state for the NH3–H2O fluid mixture: Correlation of the PVTx and vapor–liquid phase equilibrium properties | Semantic Scholar PDF] A Helmholtz free energy equation of state for the NH3–H2O fluid mixture: Correlation of the PVTx and vapor–liquid phase equilibrium properties | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/22b7325f0fe7b32b044c9bc6abc48fee497b383c/14-Figure1-1.png)



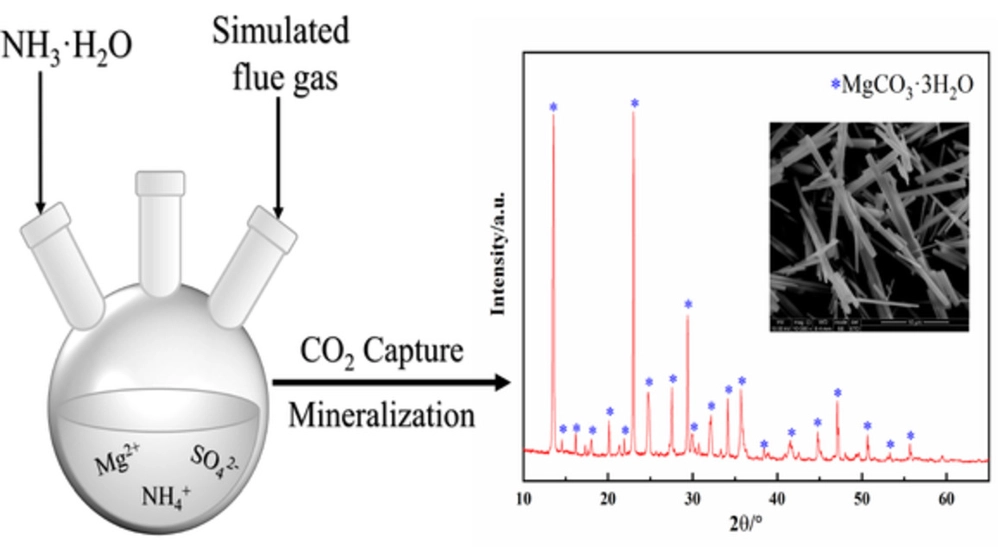
![The CO2-NH3-H2O system as described by the Thomsen model [7]. | Download Scientific Diagram The CO2-NH3-H2O system as described by the Thomsen model [7]. | Download Scientific Diagram](https://www.researchgate.net/publication/319196113/figure/fig1/AS:533643246669824@1504241872081/The-CO2-NH3-H2O-system-as-described-by-the-Thomsen-model-7.png)
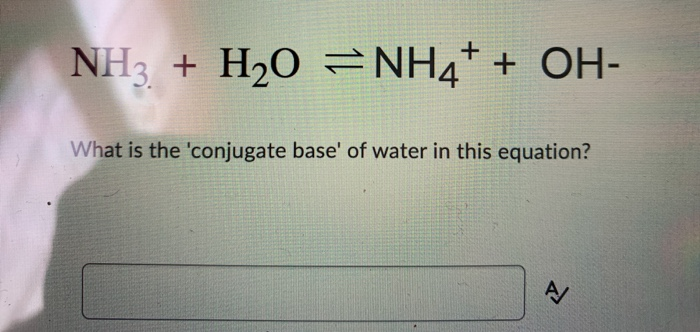



![The CO2-NH3-H2O system as described by the Thomsen model [7]. | Download Scientific Diagram The CO2-NH3-H2O system as described by the Thomsen model [7]. | Download Scientific Diagram](https://www.researchgate.net/profile/Matteo-Gazzani/publication/319196113/figure/fig1/AS:533643246669824@1504241872081/The-CO2-NH3-H2O-system-as-described-by-the-Thomsen-model-7_Q640.jpg)