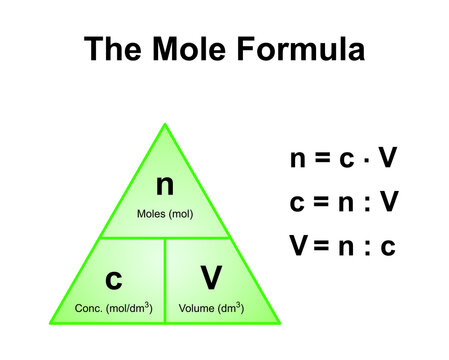
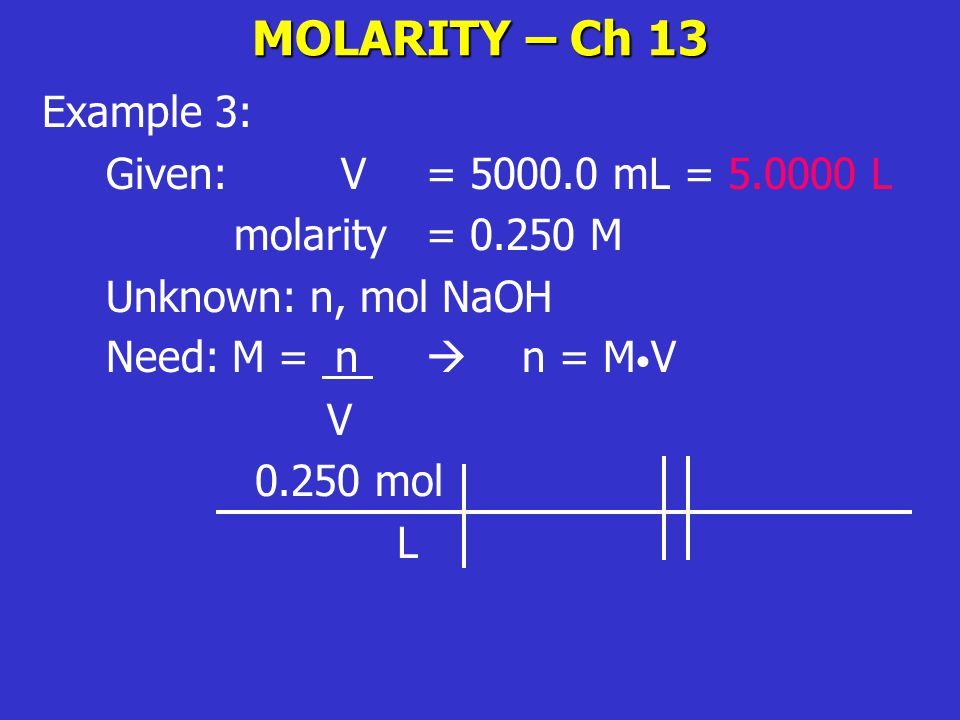
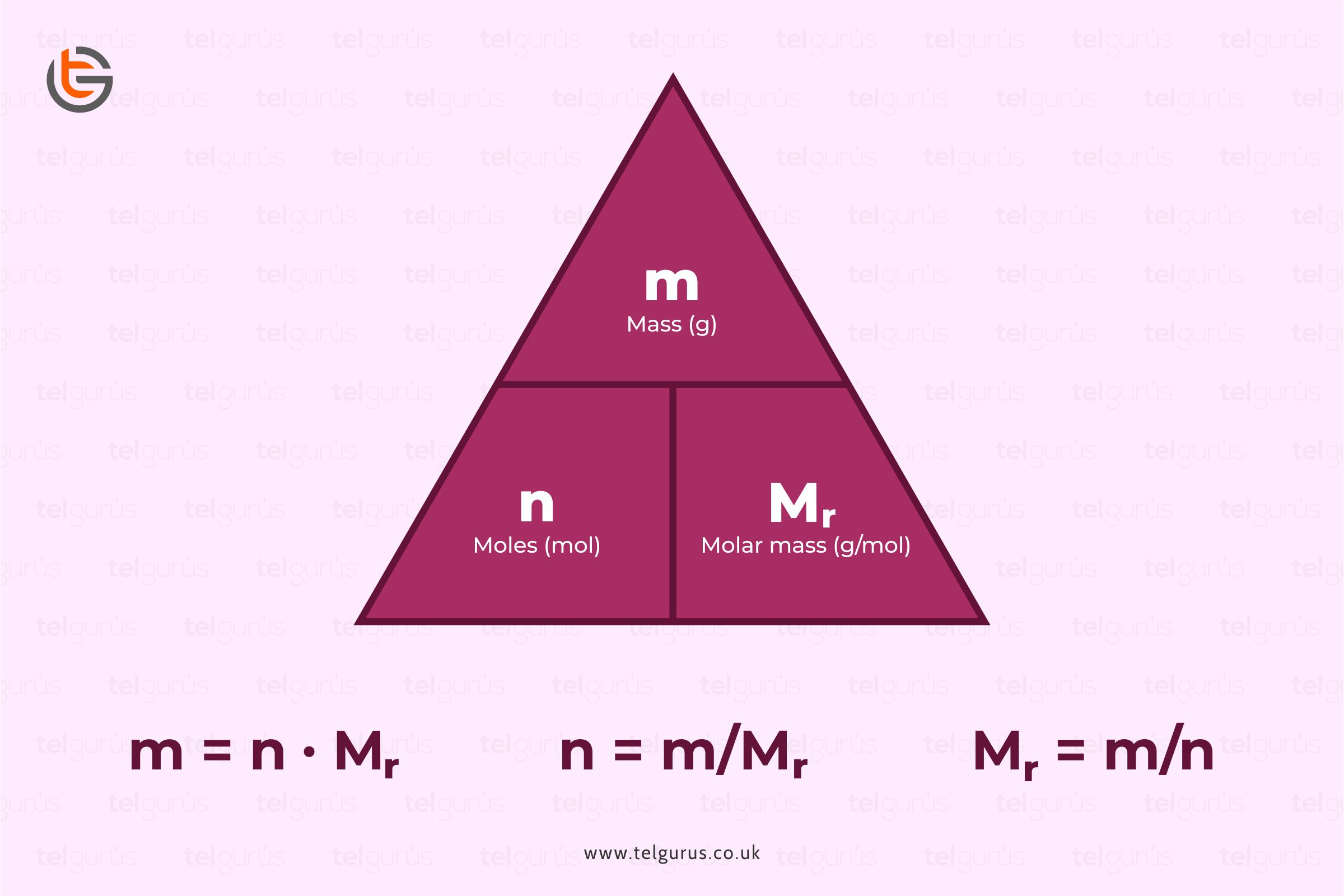
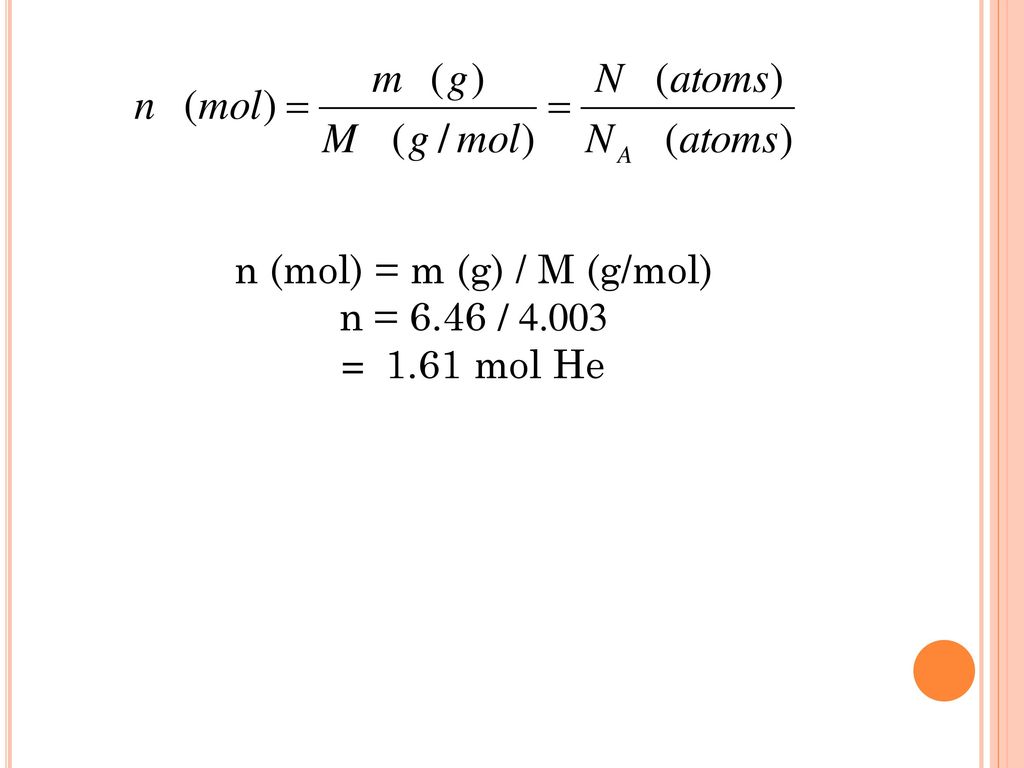The Mole And Concentration Formula Triangle Isolated On White Relationship Between Concentration Moles And Volume Cnv Stock Illustration - Download Image Now - iStock
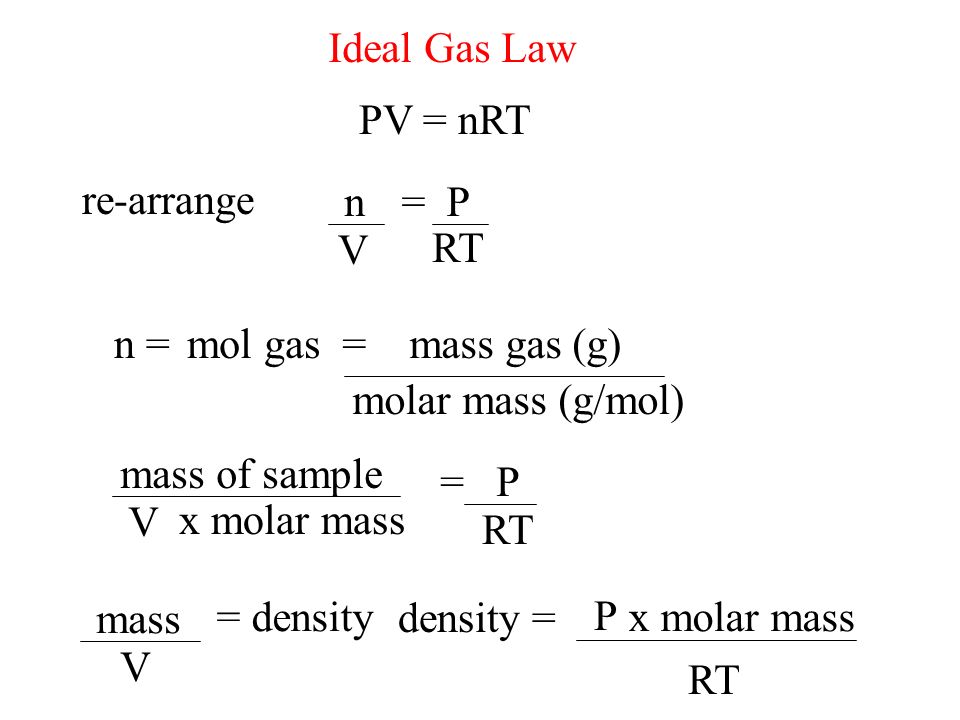
Ideal Gas Law PV = nRT re-arrange n V = P RT n = molar mass (g/mol) mol gas= mass gas (g) mass of sample V x molar mass = P RT =

The Van der Waal's equation of 'n' moles of a real gas is ( P + a/V^2 ) ( V - b ) = nRT Where P is pressure, V is volume,
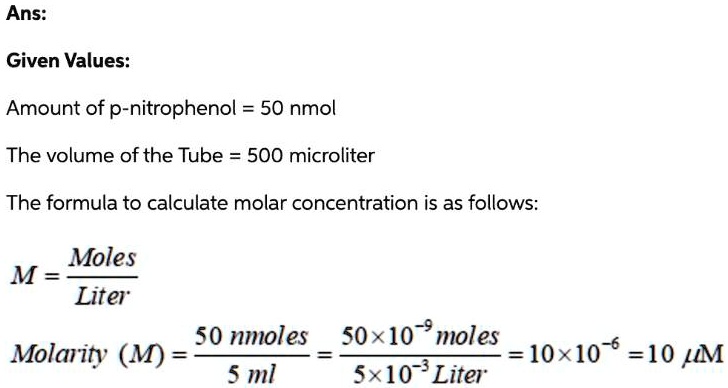
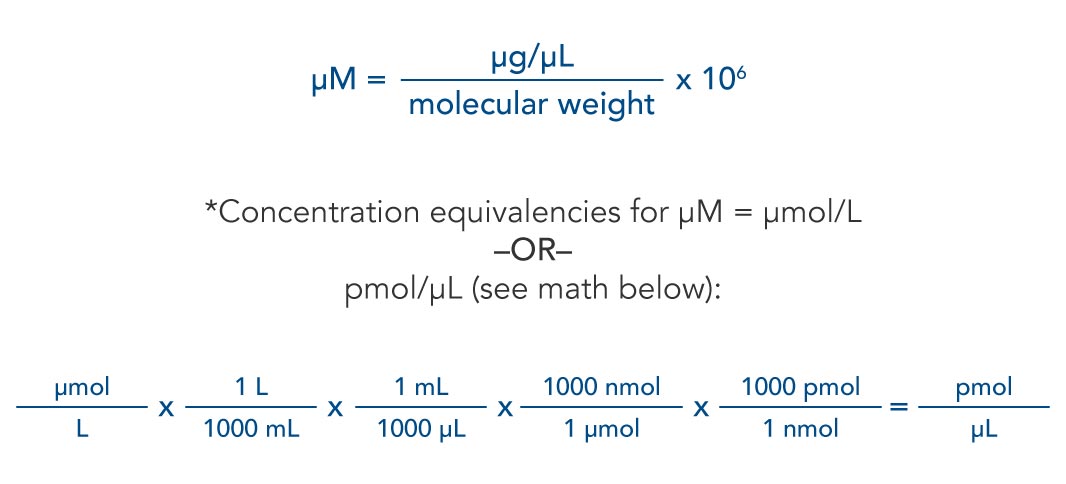
SOLVED: Ans: Given Values: Amount of p-nitrophenol = 50 nmol The volume of the Tube 500 microliter The formula to calculate molar concentration is as follows: Moles M Liter 50 nmoles S0x10-


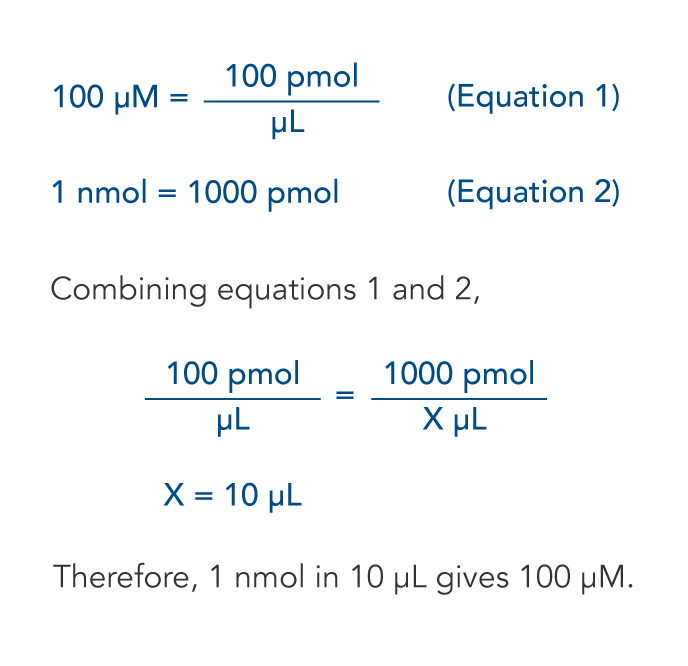
![Mol • einfach erklärt: Berechnung, Umrechnung · [mit Video] Mol • einfach erklärt: Berechnung, Umrechnung · [mit Video]](https://d3f6gjnauy613m.cloudfront.net/system/production/videos/003/617/60e8434046b2a18a42e887d5117b55f87397c228/poster_Thumbnail_Mol.jpg?1639048296)