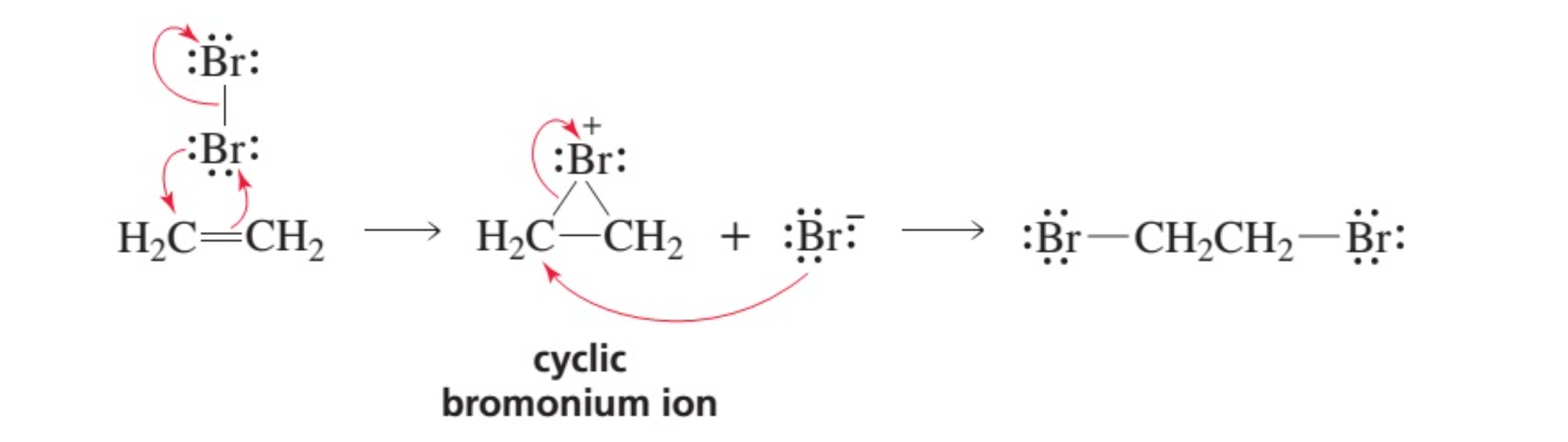
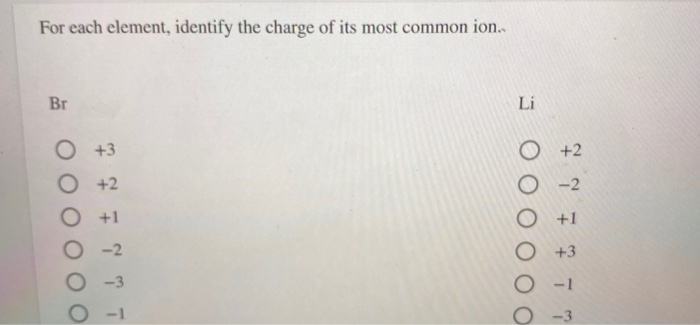
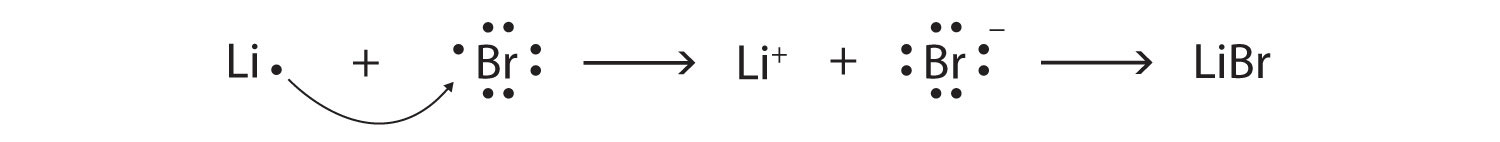Br2=BrO3^-+Br^- balance the redox reaction in an acidic medium. br2=bro3^-+ br^- @mydocumentary838 - YouTube
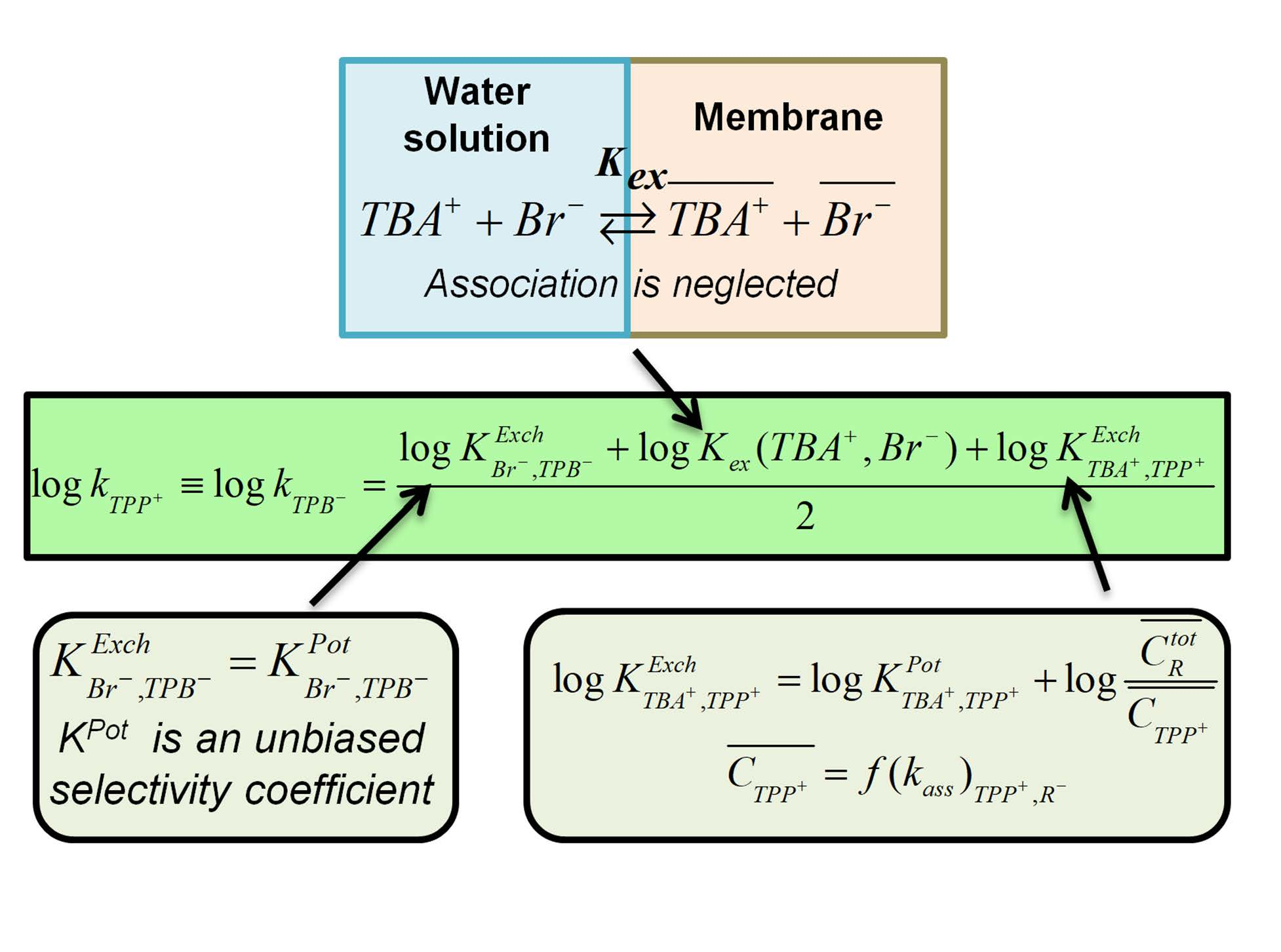
Membranes | Free Full-Text | Determination of Single-Ion Partition Coefficients between Water and Plasticized PVC Membrane Using Equilibrium-Based Techniques
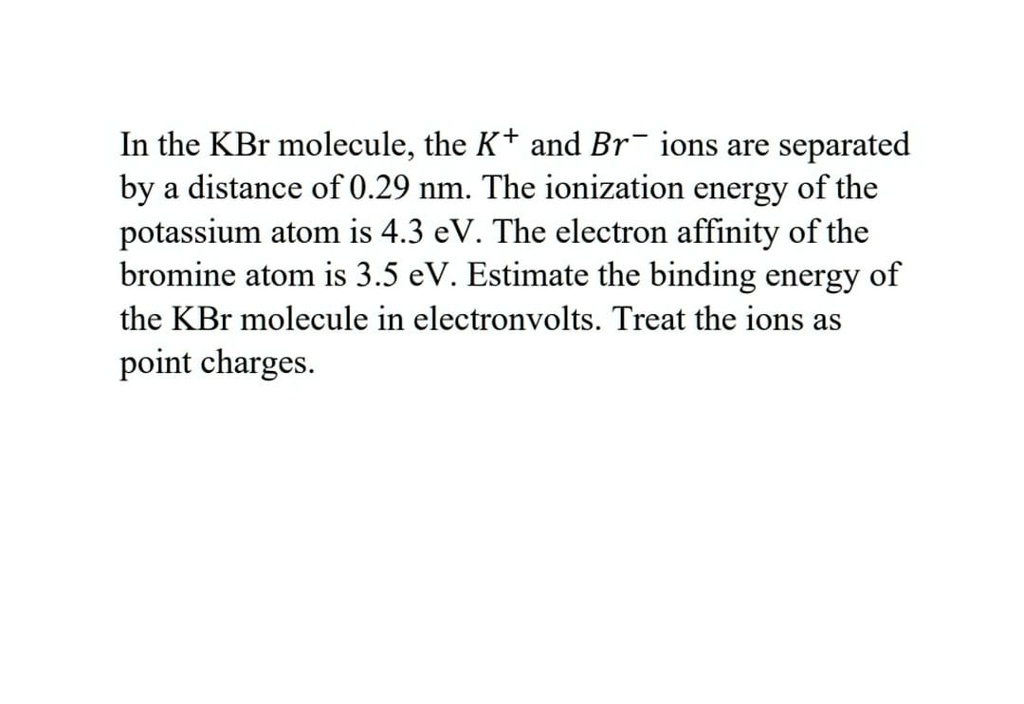
SOLVED: In the KBr molecule, the K+ and Br ions are separated by a distance of 0.29 nm: The ionization energy of the potassium atom is 4.3 eV. The electron affinity of
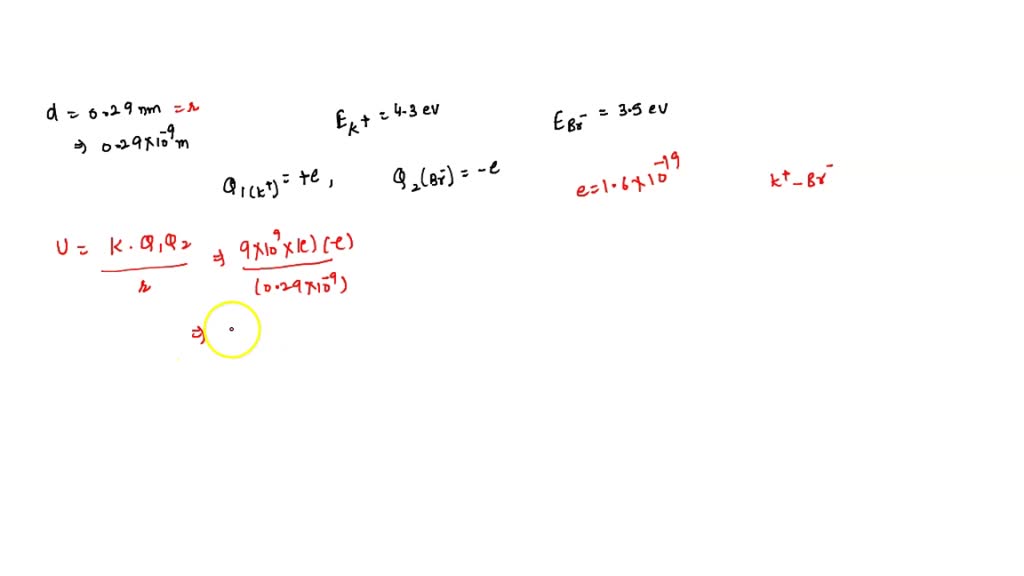
SOLVED: An Ionic Bond. (a) Calculate the electric potential energy for a K^+ ion and a Br^- ion separated by a distance of 0.29 nm, the equilibrium separation in the KBr molecule.

Use Lewis symbols to represent the transfer of electrons between the following atoms to form ions with noble gas configurations: a. Ca and Br. b. K and I. | Homework.Study.com
when BrO3^ ion react with Br^ ion in acidic medium ,Br2 is liberated.the equivalent mass of Br2 in the reaction is (M=molar mass of Br2) (1)5/3M (2)3/5M (3)4/6M (4)5/8M

Permanganate ion reacts with bromide ion in basic medium to give magnesium dioxide and bromate ion.Write the balanced ionic equation for the reaction.