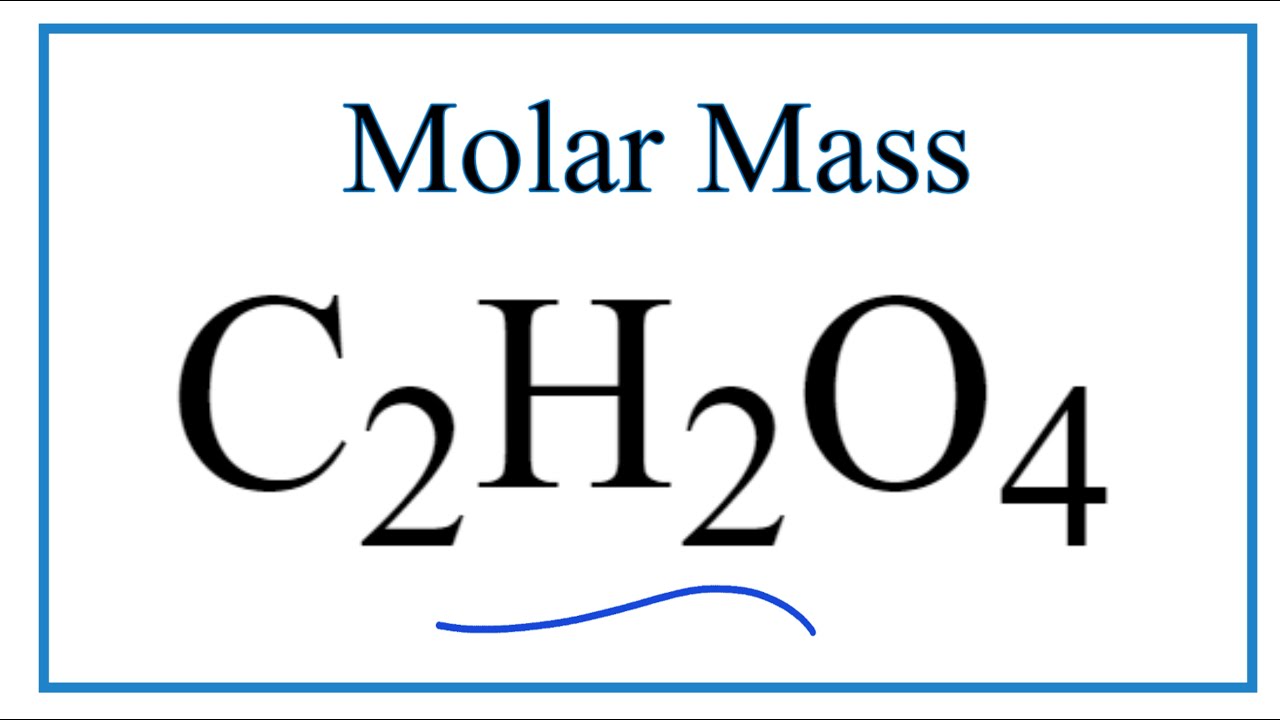H2c2o4.2h2o High Purity Oxalic Acid 99.6% - Buy Oxalic Acid 99.6%,Oxalic Acid Price,Oxalic Acid Product on Alibaba.com

Oxalic acid, H2C2O4*2H2O molar mass = 12607 g/mol is often used as a primary standard for the sta - YouTube

OneClass: how do i do question #4? Calculate the molar mass of oxalic acid dihydrate H_2C_2O_4 2H_2O....
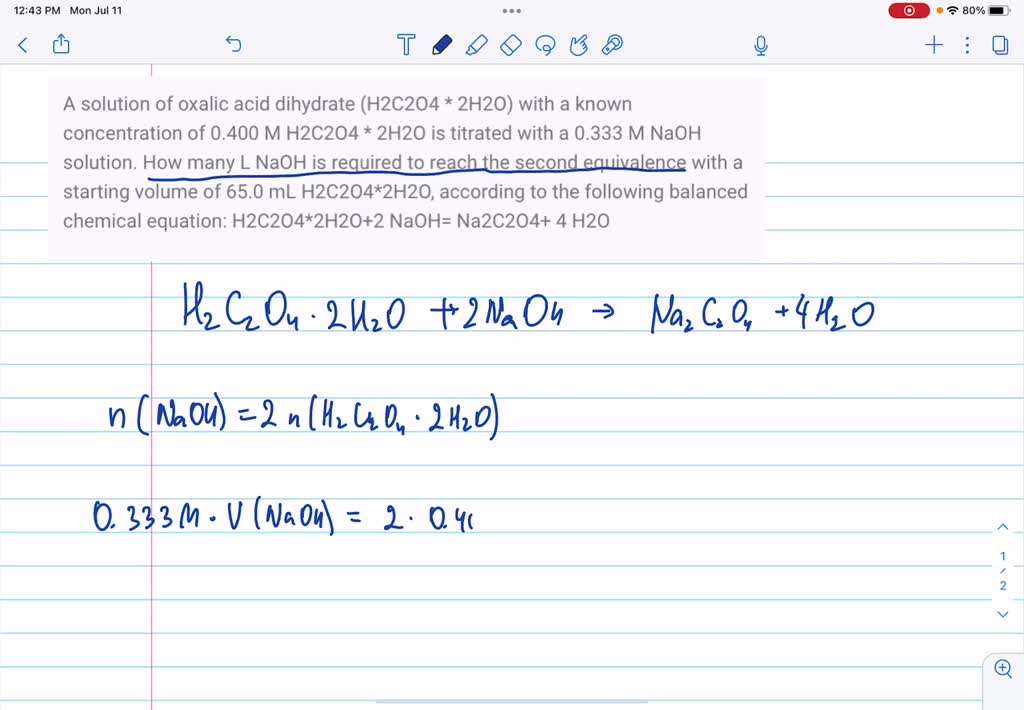
SOLVED: A solution of oxalic acid dihydrate (H2C2O4 * 2H2O) with a known concentration of 0.400 M H2C2O4 * 2H2O is titrated with a 0.333 M NaOH solution. How many L NaOH
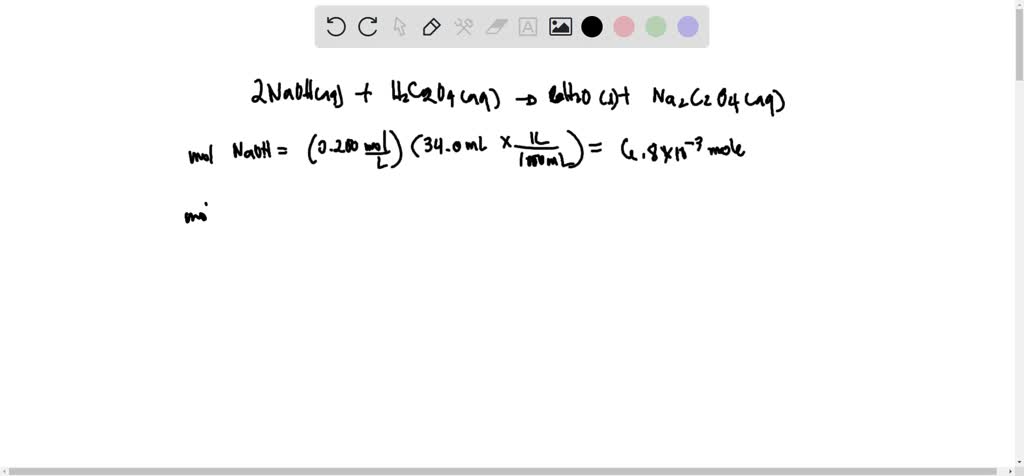
SOLVED: 2 NaOH(aq) + H2C2O4 = 2H2O(s) Na2C2O4(aq) + 4 H2O(l) Calculate the molarity of an oxalic acid solution if it takes 34.0 mL of 0.200 M NaOH solution to consume the
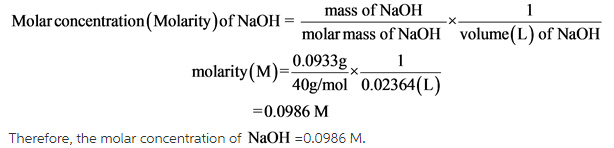
Oxalic acid, H2C2O4*2H2O (molar mass = 126.07 g/mol) is oftenused as a primary standard - Home Work Help - Learn CBSE Forum
Calculate the amount of oxalic acid (H2C2O4.2H2O) required to obtain 250 mL of deci-molar solution. - Sarthaks eConnect | Largest Online Education Community
What mass of oxalic acid dihydate, H2C2O4•2H2O, is needed to make a 0.498 M solution of oxalic acid in a 250.0 mL volumetric flask? - Quora

High Quality Oxalic Acid H2c2o4.2h2o,99.6% Min Purity - Buy Oxalic Acid H2c2o4.2h2o,Oxalic Acid C2h2o4.2h2o,Oxalic Acid H2c2o4 2 H2o Product on Alibaba.com


![Oxalic Acid Dihydrate [H2C2O4 2H2O ] [CAS_6153-56-6] 99.6+% Fine White – Wintersun Oxalic Acid Dihydrate [H2C2O4 2H2O ] [CAS_6153-56-6] 99.6+% Fine White – Wintersun](https://cdn.shopify.com/s/files/1/0724/7981/products/15-005-1_1024x1024.jpg?v=1550179687)