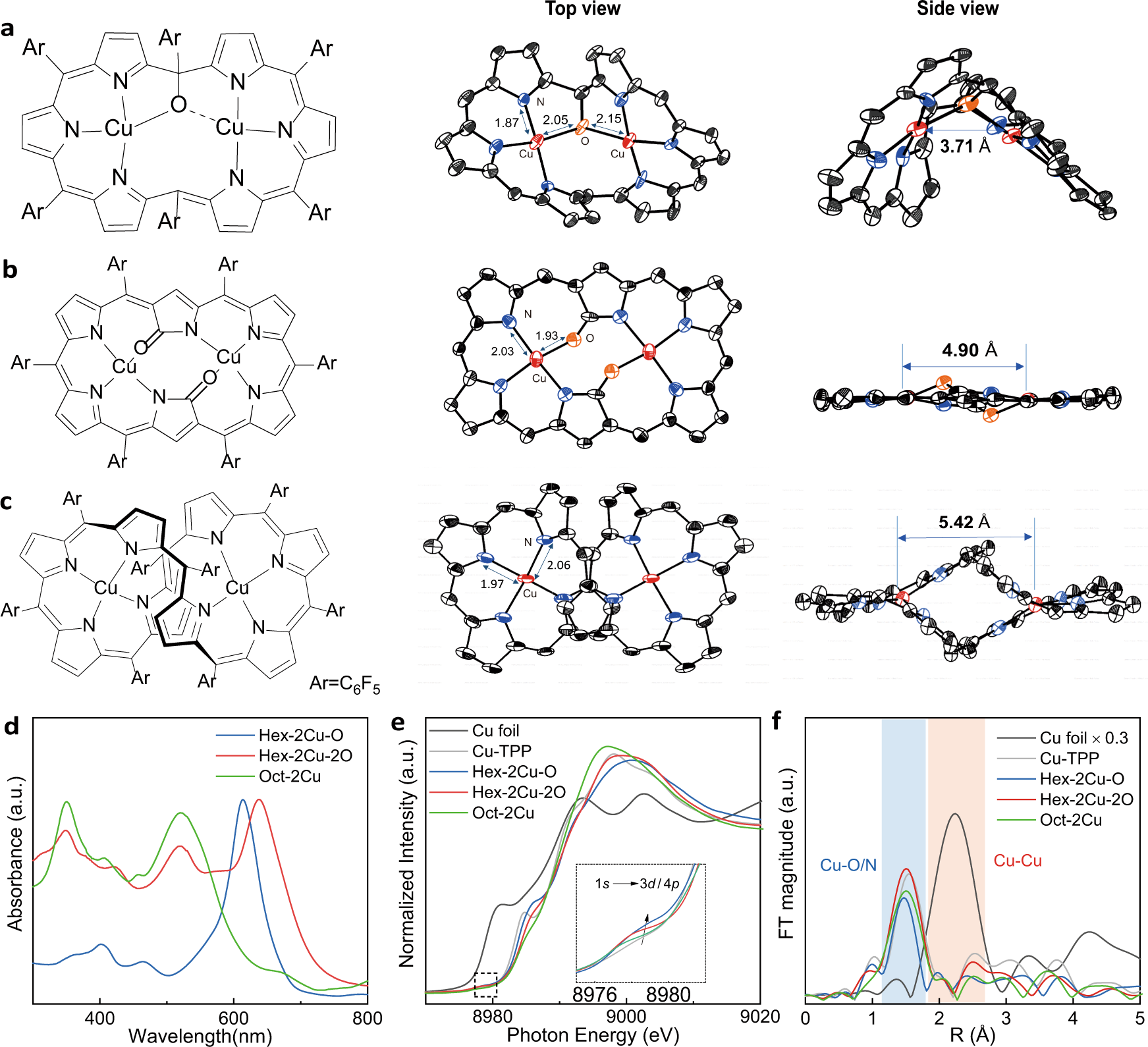
Electrocatalytic CO2 reduction to alcohols by modulating the molecular geometry and Cu coordination in bicentric copper complexes | Nature Communications

Correlating the Experimentally Determined CO Adsorption Enthalpy with the Electrochemical CO Reduction Performance on Cu Surfaces - Xiong - 2023 - Angewandte Chemie International Edition - Wiley Online Library
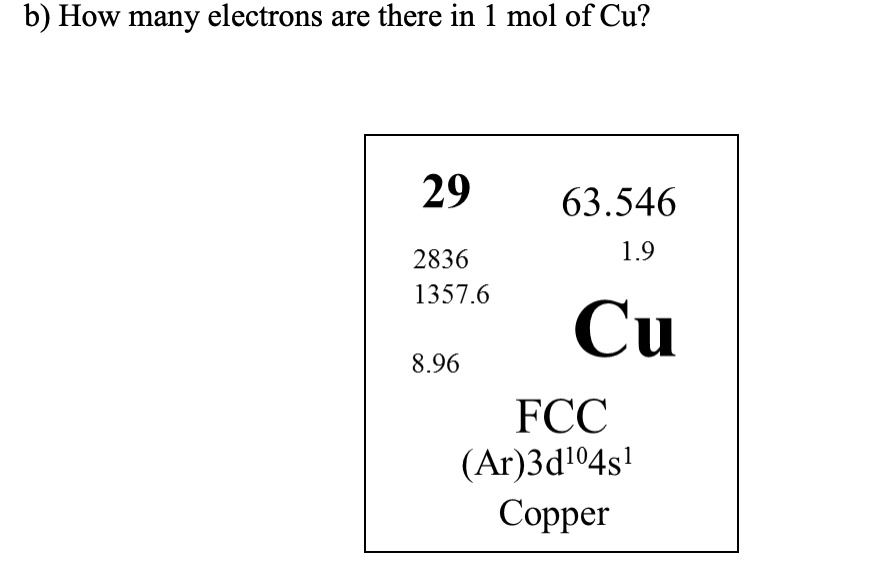
SOLVED: b) How many electrons are there in 1 mol of Cu? 29 63.546 1.9 2836 1357.6 Cu 8.96 FCC (Ar)3dl4s! Copper

Applied Sciences | Free Full-Text | Molecular Dynamics Simulation of Bulk Cu Material under Various Factors
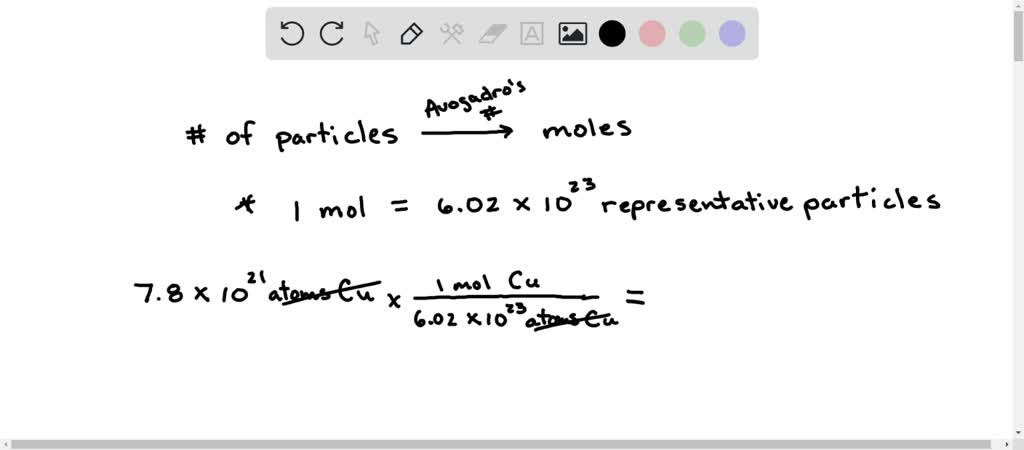
SOLVED: Calculate the number of moles of Cu in 8.9 x 1021 atoms of Cu, Express your answer using two significant figures. Azd moles of Cu Submit Previous Answers Request Answer Incorrect;

How many grams of Cu would be needed to react with 2.0 mol of HNO3? 3 Cu(s) + 8 HNO3(aq) --> 3 - Brainly.com