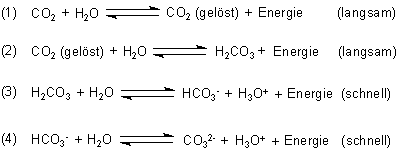Data-Driven Many-Body Models for Molecular Fluids: CO2/H2O Mixtures as a Case Study | Journal of Chemical Theory and Computation

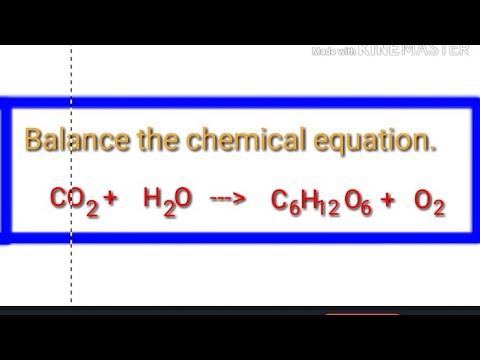
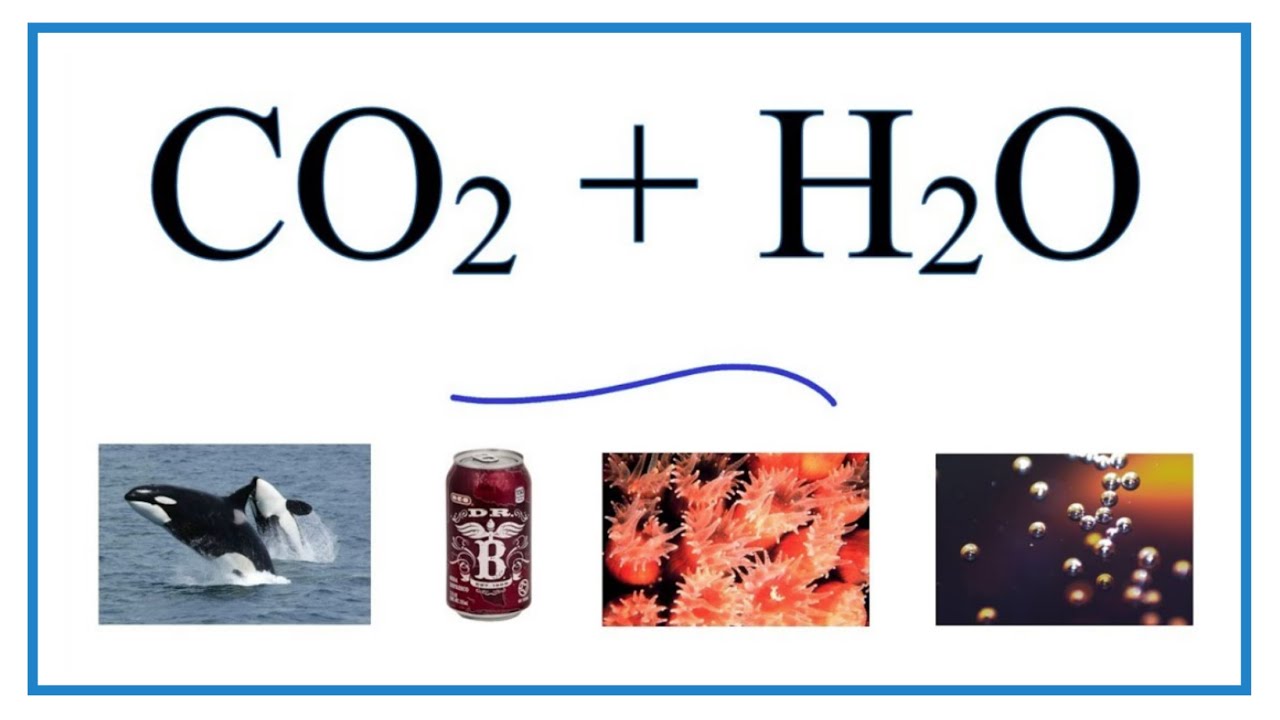
CO2+H2O=H2CO3 balance the chemical equation @mydocumentary838. co2+h2o=h2co3 balance the equation. - YouTube
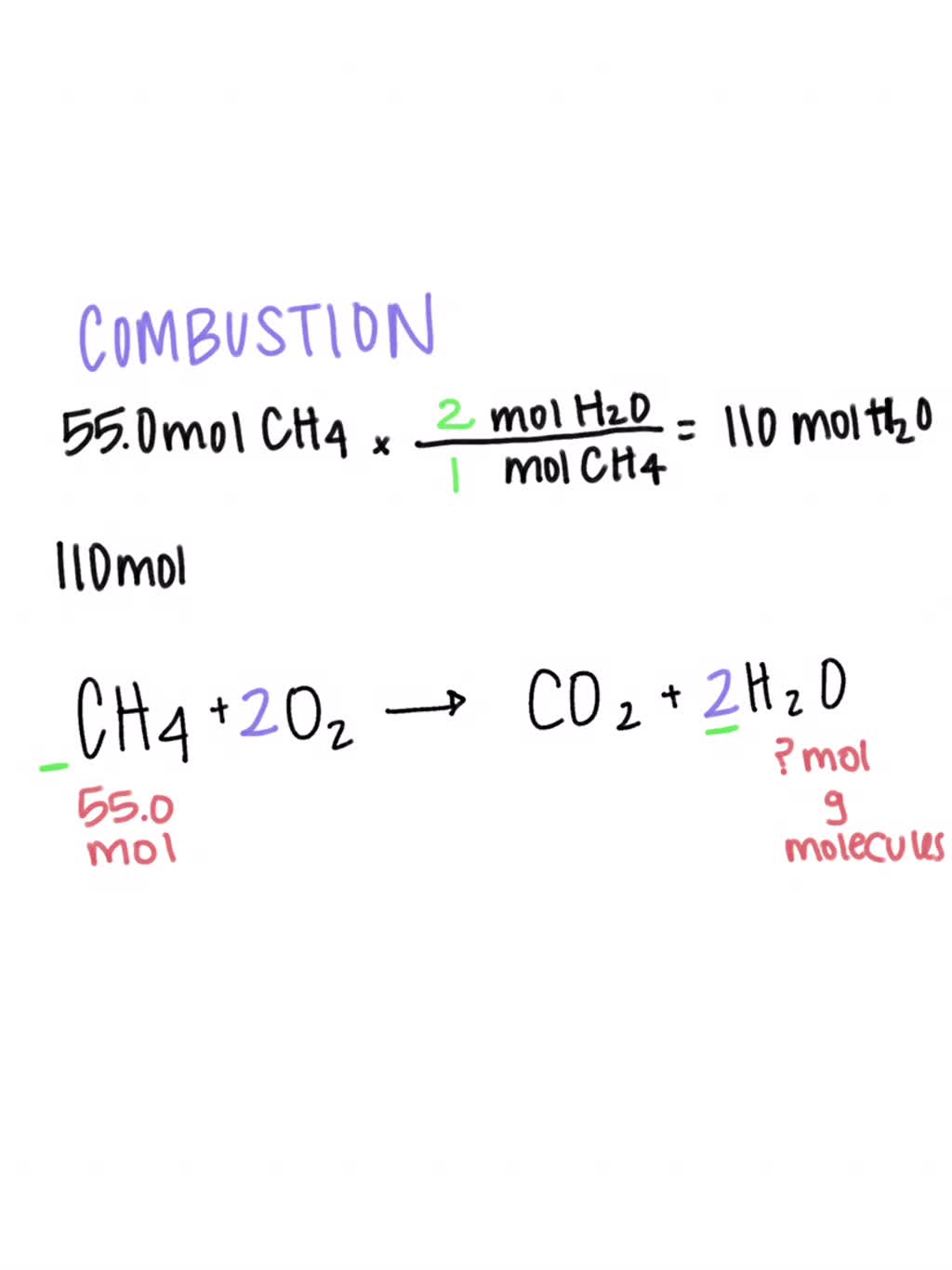
SOLVED: CH4 + O2 ——————- CO2 + H2O What type of reaction does this equation represent? Write and Balance the equation. Assume that there are 55.0moles of CH4. How many moles of
Write fully balanced equations for the following : (a) CO2 + H2O → ............ - Sarthaks eConnect | Largest Online Education Community

The promoting effects of CO2 and H2O on selective hydrogenations in CO2/H2O biphasic system - ScienceDirect